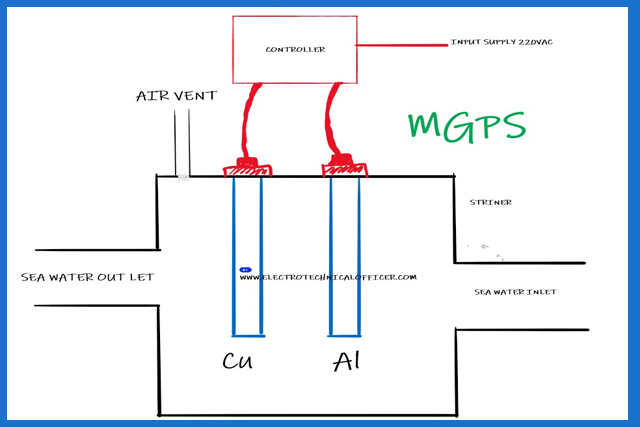
MGPS – Marine Growth Prevention System
The seawater system is protected against fouling by the anti-fouling system.
The system
protects against marine growth and corrosion by passing an electric current
through anodes placed at the seawater intakes.
The marine
growth protection anodes (Cu) are made from copper and the trap corrosion
anodes (Al) are made from aluminium alloy. The anodes are fitted in the suction
strainers.
The port
and starboard side strainers each have two MG anodes and two TC (Trap
corrosion) anodes.
A low
current must be maintained at the sea suction strainer which is not operating.
Working of MGPS
MGPS is based
on the electrolytic principle and consists of copper, and aluminium anodes
Reaction
at copper Anode:
The Cu anodes release copper ions when an electric voltage is applied. These Cu ions combine with oxygen in the water to form cuprous oxides which have a strong toxic effect on marine organisms which enter the system, thereby minimizing their growth and fouling of the seawater system.
|
Anode reaction |
Cu → Cu2 + + 2e-
|
|
Cathodic reaction |
3 H2O + 2e- → H2 + 2OH- |
|
Colloid formation |
Cu2+ + 2OH- → Cu2O |
Reaction
at Aluminium Anode:
The TC
anodes form aluminium hydroxide when an electric current is applied. This forms
an anti-corrosion barrier on the steel pipework of the seawater system.
|
Anode reaction |
Al → Al 3 + 3e-
|
|
Cathodic reaction |
3 H2o + 3e- → 3/2H2 + 3OH- |
|
Colloid formation |
Al3+ + 3OH- → Al(OH)3 |
General
Specifications of MGPS
Power
supply: 220VAC 60 Hz Maximum output 20A DC
Anodes:
Copper (Cu) and Aluminum (Al)
What is the life of MGPS anodes?
Anode life
is approximately 2.5 years.
Effects of low Current settings
Too low a
current results in insufficient protection.
Effects of High current settings
Too high a
current results in rapid wasting of the anodes.
Higher
currents than necessary will result in the rapid depletion of the anodes and
the system will not be protected when the anodes have wasted away.
The rapid
depletion of the copper anodes can result in the deposition of the copper on
the suction strainer causing partial blockage of the strainer. The condition of
the strainers and the anodes should be checked at six-monthly intervals to
ensure that this depletion is not taking place.
When do we have to reduce the Anode current?
If the seawater flow rate is reduced from this value the current applied to the anodes
should also be reduced.
Incorrect the setting of the current can result in inadequate protection against marine
growth and corrosion.
Adjustment
of current should only be made after consulting the Maker or Operating Manual.
What is the operating current of MGPS Anode?
Generally,
|
Anode |
In operation current |
Not operating current |
|
Al Anode |
3.3 A |
0.05 A |
|
Cu Anode |
1.7 A |
0.05 A |
A low current must be maintained at the sea suction strainer which is not operating.
It is
essential that the correct current is always applied to the anodes at the
operating sea water suction chest as the maker advises.
Which of the anodes decomposes faster?
Copper (Cu)
anode.
What is electrolysis?
Electrical
current flow through the liquid causes a chemical reaction.
What is the concentration of copper?
concentrations of copper are extremely small -less than 2
parts per billion
Maintenance
Check the
current readings and note them down every day.
The anodes
must be checked periodically in order to ensure that they are wasting at the
expected rate.















3 Comments
Gain lots about the mgps ,thanks for sharing
ReplyDeletehow to calculate current setting and life of MGPS electrode
ReplyDeleteIf fouling content is more than 10 ppm, how much ampere of power supply must be applied?
ReplyDeleteWe love to hear your comments on this article, so that we may better serve you in the future.